What sampling vocabulary should I know?
Pharmaceutical sampling comes with plenty of specialized terms. Here's a brief overview of some of the regulatory requirements and specialized sampling processes you might encounter.
Controlled substance sampling
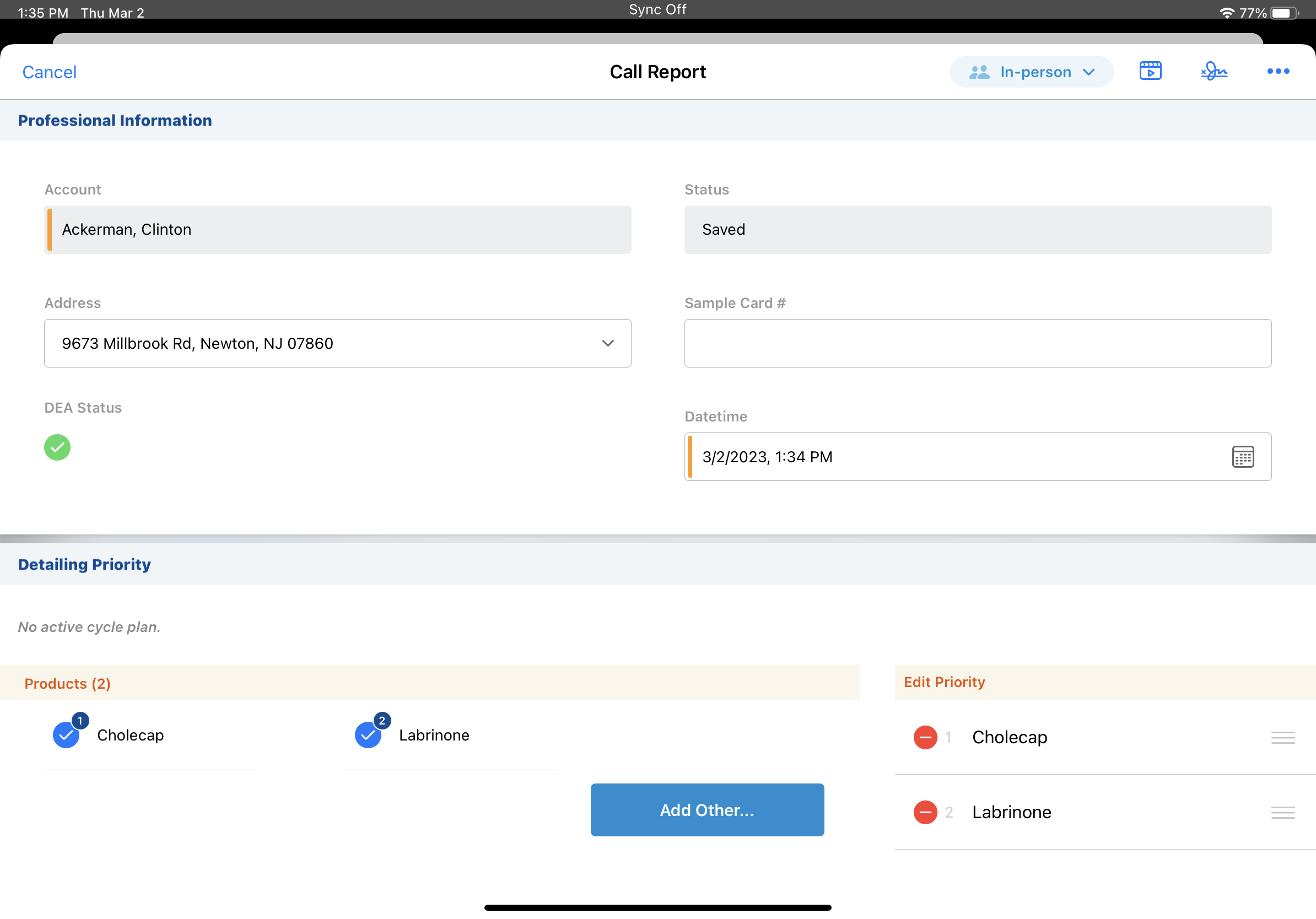
There are lots of regulations around controlled substances, which are defined and regulated by the federal Drug Enforcement Agency (DEA). Vault CRM can help you keep track of which HCPs are eligible for controlled substance sampling.
Let's say you're planning to discuss a product with an HCP, but the product happens to be a controlled substance. Before talking about this product with the HCP or offering to provide samples, you need to be sure the HCP is eligible to handle and prescribe the product. The DEA status indicator on the call report shows that the HCP has a valid DEA license for controlled substances. When you capture the HCP's signature for sampling, validation rules run to ensure the HCP is licensed for the controlled substance sample.
Prescription Drug Marketing Act (PDMA)
To help ensure patients receive safe, effective products, the Prescription Drug Marketing Act (PDMA) regulates how prescription medications are distributed and promoted in the United States.
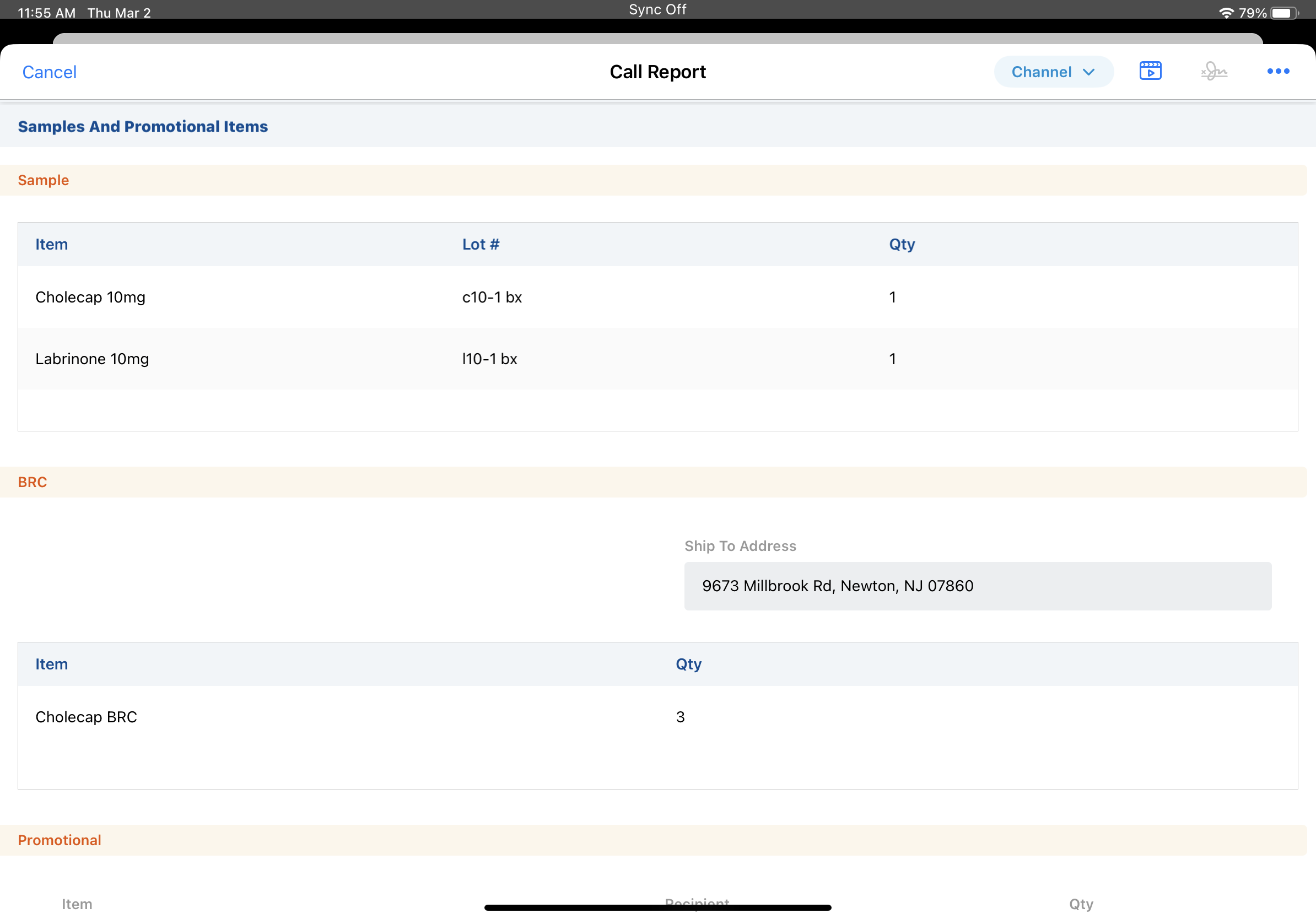
There are two key parts to the PDMA. First, pharmaceutical companies must track each sample from the time when it's manufactured to the time it's disbursed. Second, in order to receive samples, HCPs must have an active, valid license. Almost every time you provide samples to an HCP, the HCP's eligibility needs to be checked. There is one exception to this requirement: if your organization allows one-time sample drops, you don't need to validate the HCP's license the first time you sample to a new account.
Vault CRM has built-in license management and validation tools to make PDMA compliance easier. For example, when you capture an HCP's signature, all fields related to sampling are locked down on the call report.

|
You can customize Vault CRM for sampling over-the-counter medications and other products not covered by the PDMA. To learn how to enable non-PDMA sampling, see Sampling Non-PDMA Regulated Products on the Vault CRM Online Help. |
State Distributor license validation
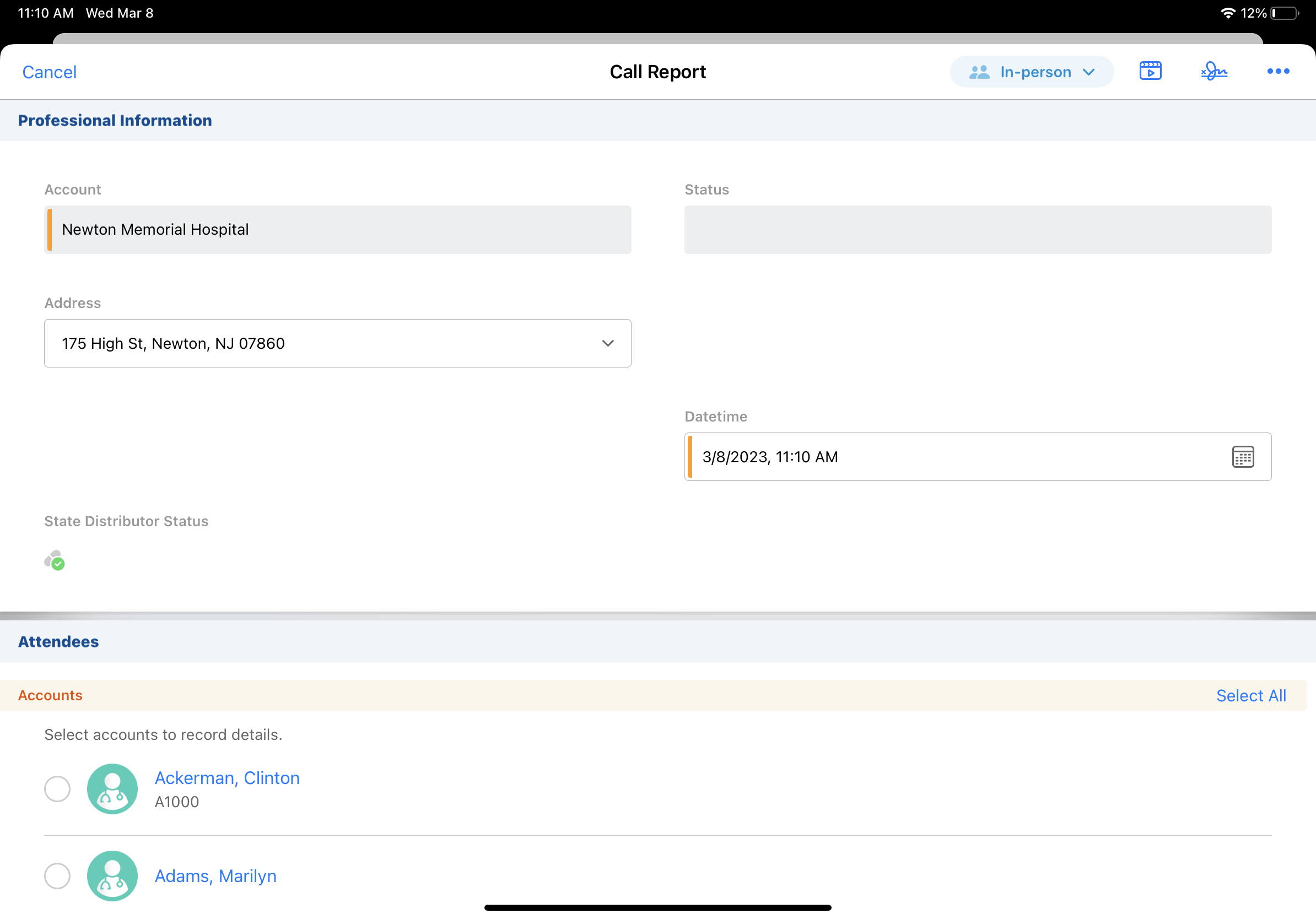
In addition to federal regulations, some states have their own State Distributor license requirements. For example, Ohio requires state-level Terminal Distributor of Dangerous Drugs (TDDD) licensing for some products, so you need to be aware of State Distributor eligibility when visiting HCPs in Ohio.
On a busy day of calls, you open the call report for the next HCP you're going to visit. The State Distributor license indicator on the call report helps you quickly see which products the HCP is eligible for. You discuss products with the HCP, including controlled substances and products covered under Ohio's TDDD regulations, and offer to provide samples. When you capture the HCP's signature, validation rules run behind the scenes to double-check the HCP is eligible for the samples.
Mid-level practitioners
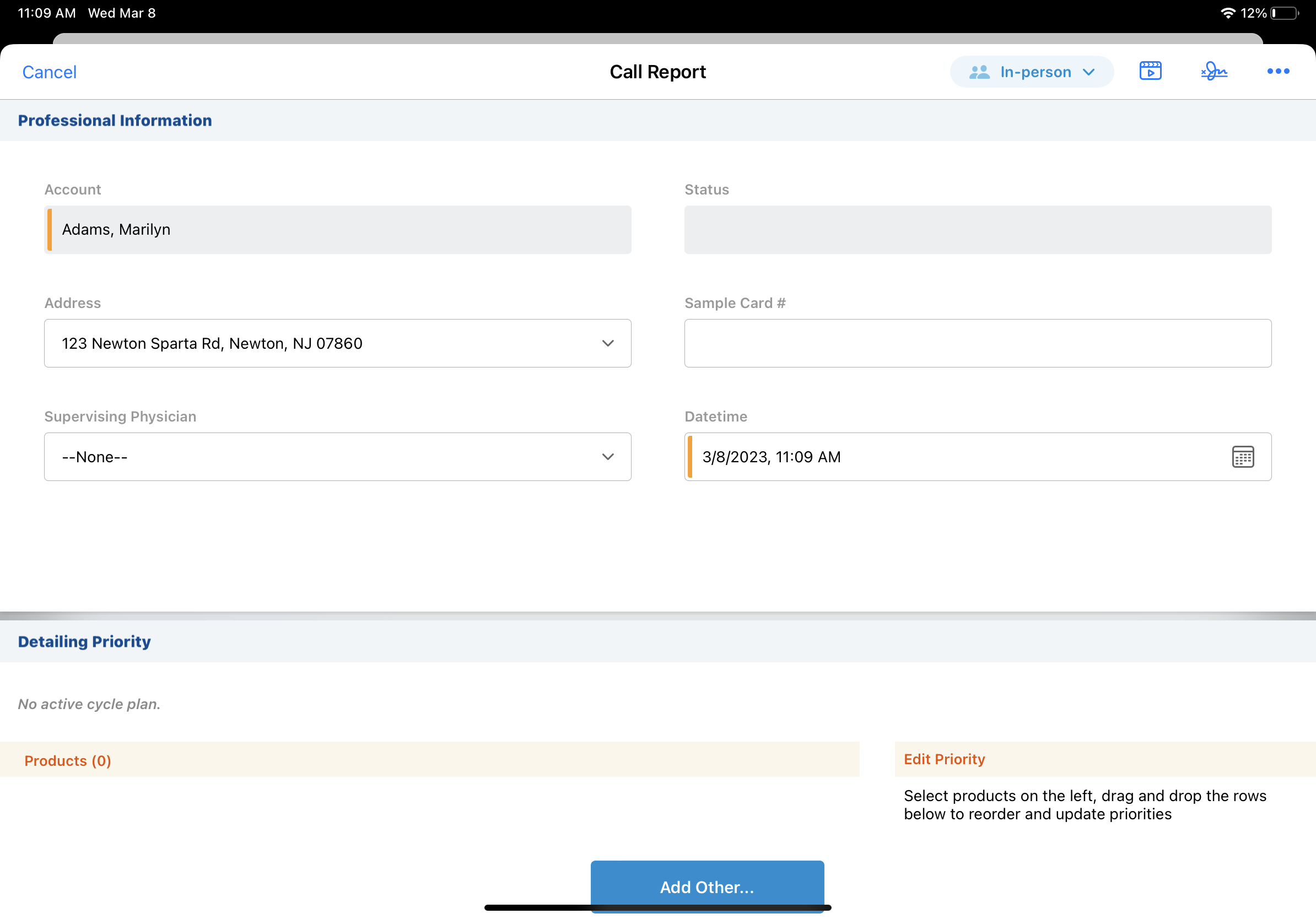
Some states allow mid-level practitioners, like nurse practitioners and physician assistants, to prescribe medications if the practitioner works with a licensed supervising physician.
For instance, you're visiting HCPs in North Carolina, and one account is a physician assistant. Because the physician assistant has a collaborative relationship with a physician, she can prescribe medications and do additional types of patient care. You discuss products with the physician assistant and offer samples. On the call report, select the physician assistant's supervising physician from the Supervising Physician pick list. When the physician assistant signs for samples, validation rules ensure her supervising physician's license is valid and she is eligible for the samples.
Cold chain sampling
Some products must be refrigerated to maintain quality. These products are known as cold chain samples, or temperature controlled samples.
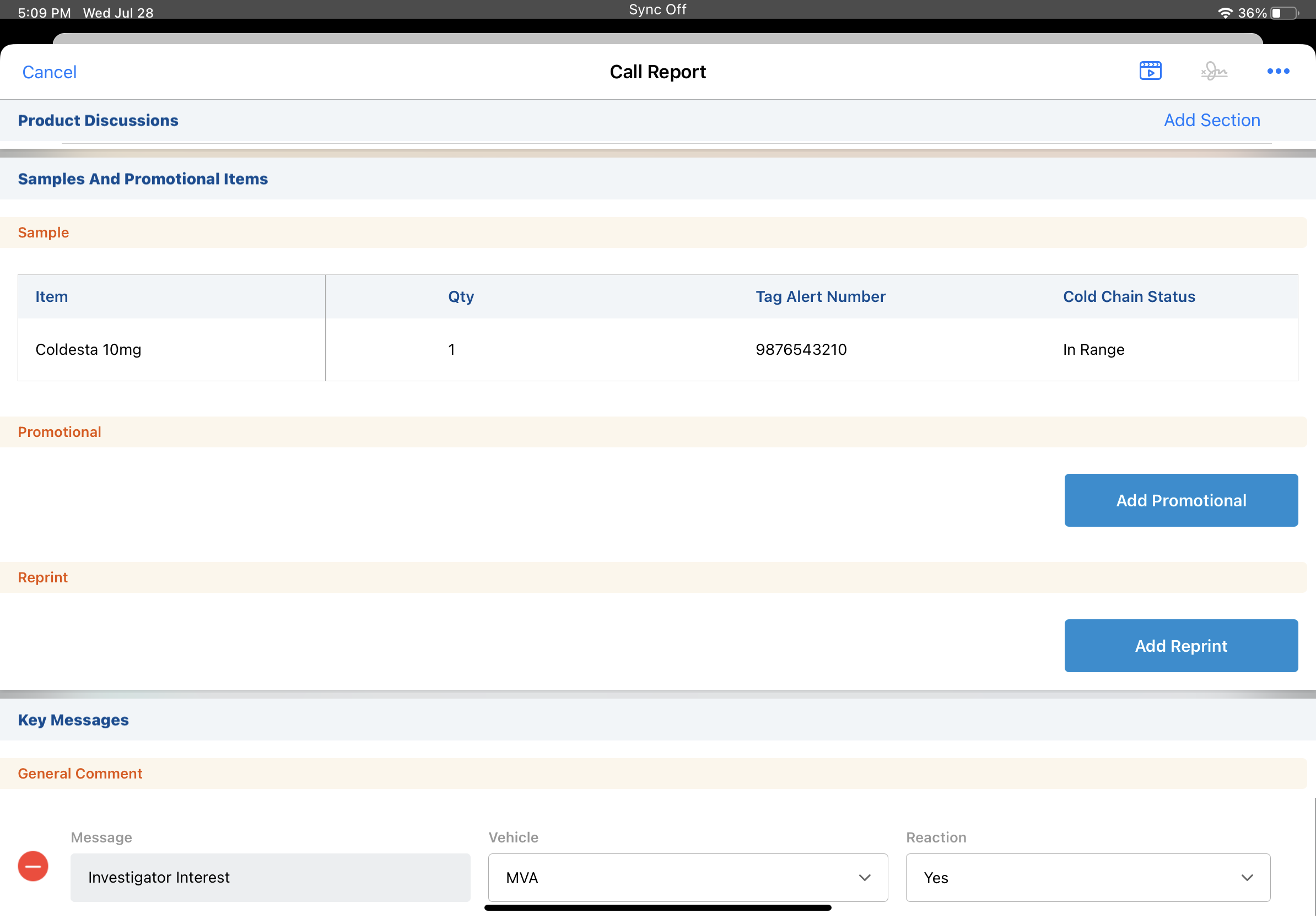
As an example, you're promoting a new product which must be kept below freezing. A temperature indicator on the sample package shows the current temperature status and a temperature indicator number, also known as the tag alert number. At each point in the sampling process, record the temperature status and the tag alert number for the cold chain sample. If the sample temperature ever exceeds the allowed temperature while under your care, it's easy to trace where and when the breach occurred.
When you first receive the samples, you enter the temperature status and tag alert number on the product receipt record. Later, you transfer a portion of the samples to another user on your team. Check the temperature indicator for each set of samples and enter the same information on the transfer record.
Finally, when disbursing samples of the product to an HCP, you check the temperature indicator again. On the call report, enter the temperature status and tag alert number for each sample. Because you recorded the temperature status and tag alert number at each step in the sampling process, you're confident the samples remain safe and effective for the HCP's practice.

|
Once you're familiar with the basics of sampling in Vault CRM, find more information on customizing sampling for specific regulatory environments on the Vault CRM Online Help. |